RESEARCH
The goal of our lab is to understand the signaling events in cancer and inflammation and to harness the extracellular vesicles/exosome machinery to engineer cellular communication and cure human diseases.
Understanding the role of exosomes in head and neck cancer progression and cancer immunomodulation
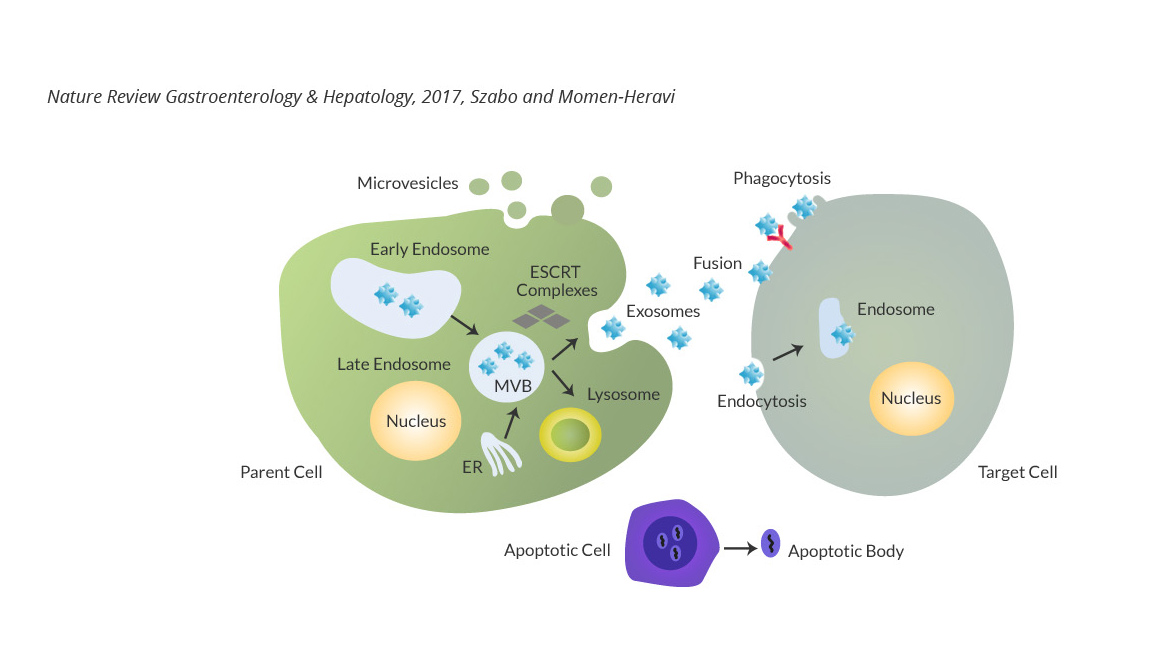
We are interested in understanding the role of exosomes/extracellular vesicles in modulating the tumor microenvironment, facilitating metastasis, and mediating cellular cross talk. We use advanced tools of biochemistry, molecular biology, and synthetic biology to identify and interrogate the key players and mechanistic principles underlying intercellular communications. Ultimately, we hope to develop a total biological understanding of how exosomes function in the pathogenesis of cancer. We are also interested in the development of novel genome editing technologies to facilitate these experiments.
Genome Engineering For Cancer Treatment
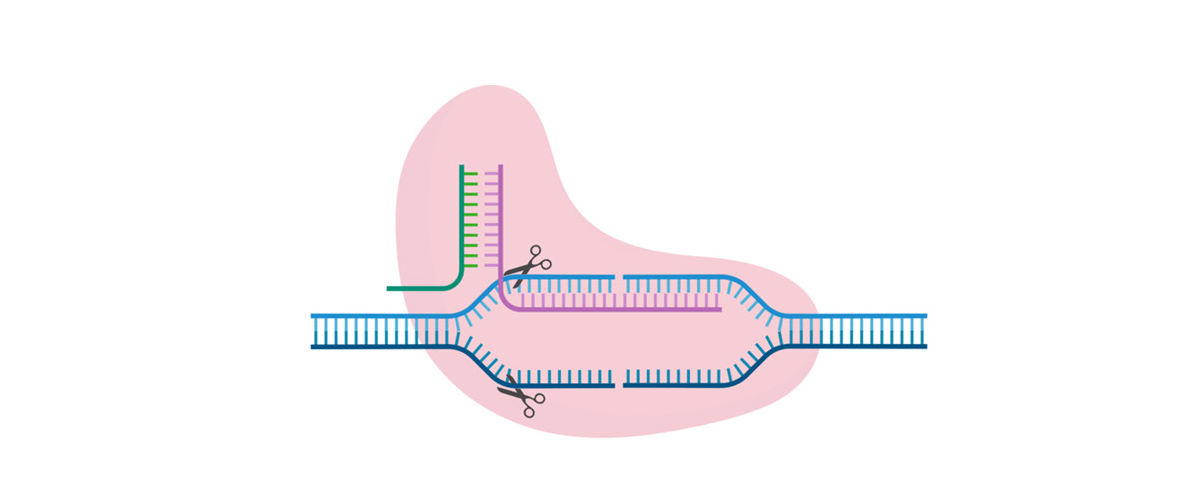
Our team utilizes the CRISPR/CAS gene editing system to edit the squamous tumors and immune system to treat cancer. In this project, we target tumor specific mutations and the key elements of tumor associated macrophages, including HIF-1α, arginase 1, and the PD-L1-PD1 pathway to reverse the tumor suppressive phenotype. The result of our work can be applicable to different tumors and activate an anti-tumor immune response.
The role of monocyte / macrophage signaling in the pathogenesis of periodontal disease and diabetes
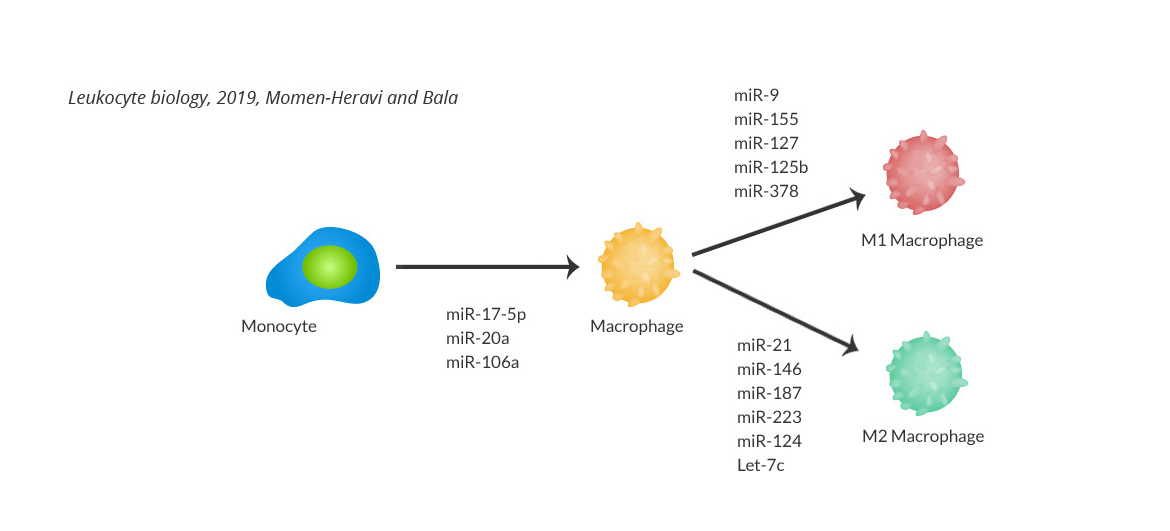
Type 2 diabetes mellitus (T2DM) and periodontitis are chronic diseases affecting millions of people globally and have long been considered biologically linked. Although pro-oxidative, pro-inflammatory and catabolic effects have been described as mechanisms underlying the enhanced periodontal bone loss in diabetes, the upstream events involving innate immunity involvement and activation are still not fully understood. Our preliminary results indicate the activation of a subset of transcription factors that are dysregulated in both diabetes and periodontal disease. This activation polarizes macrophages to a hyperinflammatory phenotype. By understanding these mechanisms and key molecular drivers, we are looking to pinpoint targetable molecules to reverse this hyper inflammatory phenotype in periodontitis with and without diabetes.
Developing biomarkers and treatment response monitoring tools for the screening of patients with solid tumors
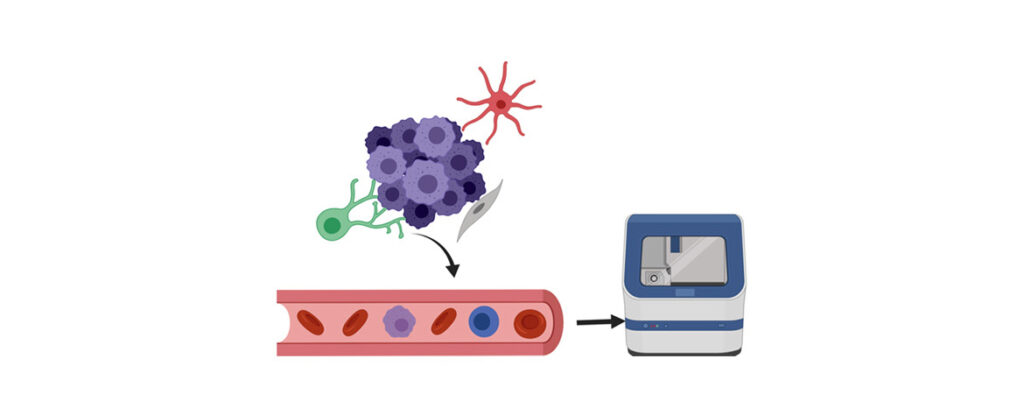
Immune checkpoint blockade therapy has shown successful clinical outcomes in the treatment of various solid tumors such as head and neck squamous cell carcinoma (HNSCC), melanoma, non- small cell lung cancer (NSCLC) and others. However, immune checkpoint inhibitors work best in patients who exhibit certain tumor biomarkers. In a collaboration with the Department of Hematology Oncology, the Department of Systems Biology, and the Mailman School of Public Health at Columbia University we aim to identify biomarkers which are associated with treatment outcome in patients with solid tumors who underwent immunotherapy
Reducing cancer health disparity by precision medicine in head and neck cancer
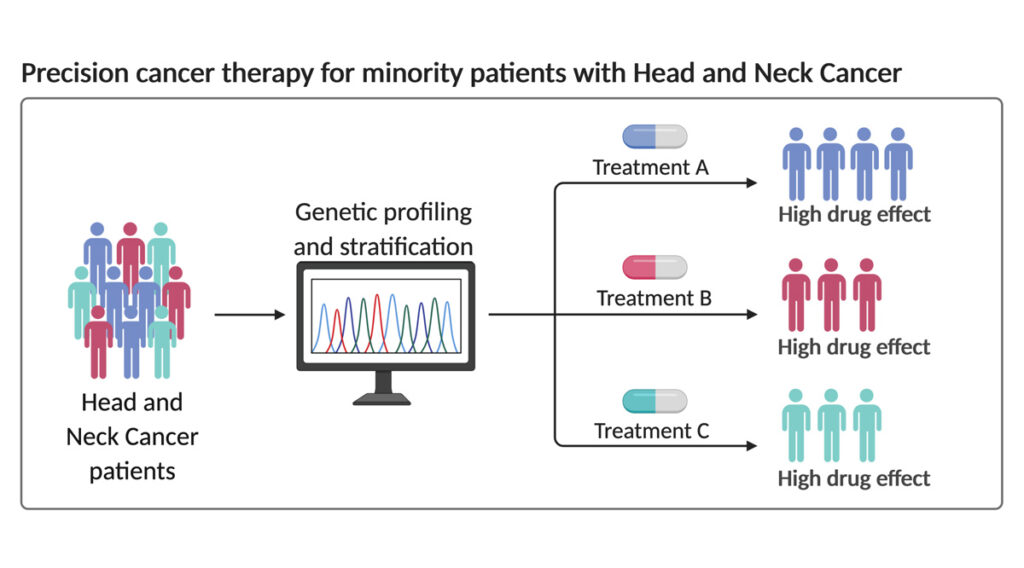
Head and neck cancer involve cancers arising from the mouth, nose, and throat regions. It is one of the most common cancer types worldwide and accounts for over 300,000 deaths each year. Furthermore, there continues to be about a 50% 5-year survival rate even after surgery. This poor prognosis is due to head and neck cancer’s tendency to both reappear, termed ‘recurrence’, and spread to other parts of the body, termed ‘metastasis’.
Currently, many new drugs are being developed in hopes of improving the recurrence and metastasis rates seen in head and neck cancers. However, epidemiological data demonstrates another urgent matter that has not been well addressed: healthcare disparities exist between racial groups in terms of screening, detection, treatment, and survival for head and neck cancer.
Specifically, the black population shows higher incidence rates of head and neck cancer than the white population. Black patients initially present with more advanced cancers than white patients. Black patients also show a worst 5-year survival rate in all types of head and neck cancer. Lastly, mortality rates are also worse in black versus white patients. Our lab aims to uncover some of the molecular uniqueness that may be present in different racial groups and use them to guide precision therapy for efficient treatment of head and neck cancer.
Developing an engineered exosome platform, for targeted and cell-specific drug delivery for treatment of human diseases
We are utilizing the body’s natural transport system as a delivery platform for targeted gene editing in the lung for the treatment of cancers and genetic diseases. Exosomes are small vesicles shed by all cells in the cellular microenvironment which carry and deliver biomacromolecules.
We have recently developed engineered exosomes which are safe and do not have any unwanted side effects with the capacity to directly target the cancer-specific mutations with CRISPR/Cas technology (SafeEXO-CAS). These SafeEXO-CAS have lung-specific targeting moieties on their surface and are able to carry endogenous active Cas9 proteins and sgRNA targeting the specific mutation in lung epithelial cells for treatment of lung squamous cells.
We are expanding this platform to target other lung diseases such as cystic fibrosis, in parallel with generating SafeEXO-CAS that can target liver, brain, and cancer cells for treatment of variety of different diseases.

